Have you ever come across a grower complaining about a pesticide that did not properly control the pest infestation at hand? You probably attributed the reduction or lack of control to either a faulty chemical batch, poor application method, or pest resistance. Have you ever thought of checking the pH of the water prior to mixing the chemical?
When inspecting any given pesticide label, chances are you will find a statement cautioning you against mixing the pesticide with alkaline materials such as lime or lime sulfur. The reason is that many pesticides, particularly organophosphate insecticides, undergo a chemical reaction in the presence of alkaline materials, leading to the annulment of their effectiveness. This reaction is called alkaline hydrolysis and occurs when the pesticide is mixed with water with a pH greater than 7. The more alkaline the water, the more rapid the breakdown of the pesticides.
Lime and lime sulfur are often mentioned on pesticide labels because they may be added to spray tanks. However, they are not the only materials that provide sufficient alkalinity for this reaction to occur. Caustic soda, caustic potash, soda ash, magnesia or dolomitic lime, and liquid ammonia provide alkaline conditions in which susceptible pesticides can be hydrolyzed into inactive organic compounds.
It was recently shown that, in many areas, water supplies have sufficient natural alkalinity to cause hydrolysis of certain pesticides. In practical terms, pesticide may begin to break down as soon as it is added to the tank. This means that the degree of pest control may be somewhat less than desirable, or even nonexistent because a certain amount of the active ingredient will be decomposed to an inactive form before it ever reaches the plant and the pest. In addition, if a spray rig stands for several hours or overnight before spraying out the contents of the tank, as much as 50 percent or more of the active ingredient may be decomposed.
Lower the pH in Our Spray Tank
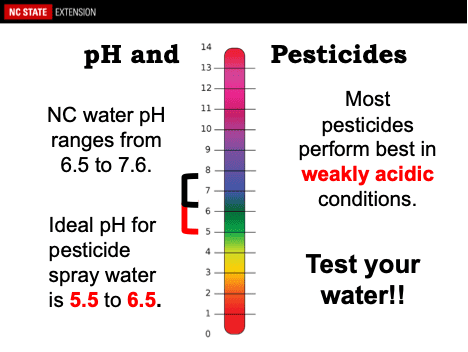
If your water supply is alkaline, especially with a pH of 8 or greater, and you are using pesticides sensitive to hydrolysis, you should lower the pH of the water in the spray tank. A pH within the range of 4 to 6 is recommended for most pesticide sprays. You can adjust your spray solution to this optimal range by using adjuvants that are marketed as buffering agents.
On another hand, some pesticide materials should not be acidified under any circumstance. Pesticides containing fixed copper fungicides (including Bordeaux mixture, copper oxide, basic copper sulfate, copper hydroxide, etc.) and lime or lime sulfur belong to this category not to be acidified. However, if the product label specifies avoiding alkaline materials, chances are that the spray mixture will benefit if the pH to 6 or slightly lower.
Which Pesticides Are Affected by Alkaline Water?
While there is a great deal of variability, insecticides are affected more severely by alkaline water than fungicides and herbicides. Among the insecticides, organophosphates and carbamates are decomposed much more rapidly than chlorinated hydrocarbons. Many manufacturers provide information on the rate at which their products hydrolyze. This rate is usually expressed as “half-life” or the “time it takes for 50 percent hydrolysis or breakdown to occur.” With trichlorfon or DYLOX, for example, the time for 50 percent hydrolysis at pH 8.0 is 63 minutes; at pH 7.0, 50 percent breakdown occurs in 386 minutes; and pH 6, 80 hours. This means that if the pH of your spray water is 8, and one hour elapses between the time you add the insecticide to your spray tank and the spray dries on the foliage, 50 percent of the active ingredient has already decomposed. But if your water has a pH of 6, it is not likely that you will lose any significant activity during the process of application.

Next time you are mixing pesticides, keep a close eye on the water you are using and always read the instructions on the label for maximum efficacy of the product.

